Consider the reaction:
2 CO(g) + O2(g)  2 CO2(g)
2 CO2(g)
The equilibrium expression for this reaction is:
A) 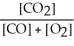
B) 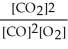
C) 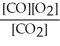
D) 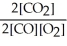
E) 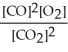
Correct Answer:
Verified
Q68: 2 SO2(g)+ O2(g) Q69: Write the equilibrium expression for the following Q70: Which change to this reaction system would Q71: The position of the equilibrium for a Q72: Match the following. Q74: Match the following. Q75: Diatomic nitrogen is added to the equilibrium Q76: Which of the following conditions characterizes a Q77: If we add a catalyst to the Q78: Given the following reaction,the equilibrium expression will![]()
-A process or reaction which
-A process or reaction that
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents