2 SO2(g) + O2(g)  2 SO3(g) + heat K = 4.8 ×
2 SO3(g) + heat K = 4.8 × 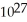
Which statement about this system is not true?
A) At equilibrium 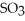
Is the predominant substance.
B) Heating the system will cause breakdown of 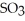
.
C) Adding 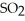
Will cause an increase in the amount of
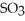
.
D) Removing 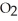
Will cause an increase in the amount of
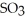
.
E) The large value of K means that the reaction essentially goes to completion.
Correct Answer:
Verified
Q63: Calculate the equilibrium constant for the reaction
Q64: Which process is not likely to be
Q65: Match the following.
-A process or reaction which
Q66: Match the following.
-A process or reaction that
Q67: Consider the reaction:
A + 2 B
Q69: Write the equilibrium expression for the following
Q70: Which change to this reaction system would
Q71: The position of the equilibrium for a
Q72: Match the following.
-A process or reaction which
Q73: Consider the reaction:
2 CO(g)+ O2(g) 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents