The first induced nuclear reaction, 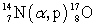 , in a laboratory was studied by Rutherford in 1919. How much energy is absorbed in this reaction if the atomic masses are:
, in a laboratory was studied by Rutherford in 1919. How much energy is absorbed in this reaction if the atomic masses are: 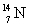 = 14.003 074 u,
= 14.003 074 u, 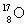 = 16.999 133 u, = 4.002 603 u, and p = 1.007 825 u? Note: 1 u = 931.5 MeV.
= 16.999 133 u, = 4.002 603 u, and p = 1.007 825 u? Note: 1 u = 931.5 MeV.
A) 1.193 MeV
B) 3.338 MeV
C) 6.603 MeV
D) 15.08 MeV
E) 27.91 MeV
Correct Answer:
Verified
Q6: What radiation absorbed dose of slow neutrons
Q7: Which one of the following quantities is
Q8: Determine the atomic number Z and the
Q9: What is the importance of thermal neutrons
Q10: A radiologist absorbs 4.0 × 10-5 J
Q12: Complete the following statement: The term ionizing
Q13: What absorbed dose of protons with an
Q14: A beam of 4.5-MeV neutrons is directed
Q15: A physicist wishes to measure the exposure
Q16: A medical researcher wishes to compare the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents