Multiple Choice
Consider the following reaction: Ni(CO) 4(g) 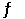 Ni(s) + 4CO(g)
Ni(s) + 4CO(g)
If the initial concentration of Ni(CO) 4(g) is 1.0 M,and x is the equilibrium concentration of CO(g) ,what is the correct equilibrium relation?
A) Kc = X4/(1.0 - 4X)
B) Kc = X/(1.0 - X/4)
C) Kc = X4/(1.0 -X/4)
D) Kc = X5/(1.0-X/4)
E) Kc = 4X/(1.0 - 4X)
Correct Answer:
Verified
Related Questions
Q43: A mixture consisting of 0.250 M N2(g)and
Q44: A mixture consisting of 0.250 M N2(g)and


