Consider the reaction PCl5(g) 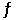 PCl3(g) + Cl2(g)
PCl3(g) + Cl2(g)
At a certain temperature,if the initial concentration of PCl5(g) is 3.0 M,at equilibrium the concentration of Cl2(g) is 0.80 M.Calculate the value of Kc at this temperature.
A) 0.21
B) 0.29
C) 0.64
D) 3.4
E) 0.46
Correct Answer:
Verified
Q40: What is the relationship between K and
Q42: For the reaction NH3(g)+ H2S(g) 
Q43: A mixture consisting of 0.250 M N2(g)and
Q44: A mixture consisting of 0.250 M N2(g)and
Q46: Consider the following reaction at a certain
Q47: Consider the reaction Q48: Consider the following reaction: Ni(CO)4(g) Q49: For the reaction 2CaSO4(s) Q50: Consider the reaction Q136: Which of the following statements is true?
N2(g)3H2(g) ![]()

![]()
Na+(g)+ Cl - (g)
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents