For the reaction NH3(g) + H2S(g) 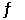 NH4HS(s)
NH4HS(s)
Kc = 9.7 at 900 K.If the initial concentrations of NH3(g) and H2S(g) are 2.0 M,what is the equilibrium concentration of NH3(g) ?
A) 1.9 M
B) 1.7 M
C) 0.20 M
D) 0.10 M
E) 0.32 M
Correct Answer:
Verified
Q37: At 25
Q38: What is the relationship between K and
Q39: At 600
Q40: What is the relationship between K and
Q43: A mixture consisting of 0.250 M N2(g)and
Q44: A mixture consisting of 0.250 M N2(g)and
Q45: Consider the reaction PCl5(g) Q46: Consider the following reaction at a certain Q47: Consider the reaction Q136: Which of the following statements is true?![]()
N2(g)3H2(g) ![]()
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents