Which of the following reactions is not affected by an increase in pressure?
A) NH4HS(s) 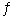 NH3(g) + H2S(g)
NH3(g) + H2S(g)
B) C(s) + H2O(g) 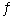 CO(g) + H2(g)
CO(g) + H2(g)
C) H2(g) + I2(s) 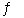 2HI(g)
2HI(g)
D) NH3(g) + HCl(g 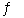 NH4Cl(s)
NH4Cl(s)
E) 2BrCl(g) 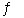 Br2(g) + Cl2(g)
Br2(g) + Cl2(g)
Correct Answer:
Verified
Q67: The equilibrium constant K for the
Q77: Consider the reaction 4NH3(g)+ 3O2(g) 
Q83: For the reaction Br2(g) 
Q85: Consider the following reaction at a certain
Q91: Calculate the vapor pressure of ethyl alcohol,C2H5OH(l),at
Q164: An endothermic reaction is most likely
Q170: If a reaction mixture that is
Q171: For the decomposition of ammonia to nitrogen
Q175: Consider the reaction
2SO2(g)+O2(g)
Q190: Calculate the standard free energy of formation
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents