Consider the reaction 4NH3(g) + 3O2(g) 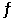 2N2(g) + 6H2O(g) ,K = 1080 at a certain temperature.
2N2(g) + 6H2O(g) ,K = 1080 at a certain temperature.
Initially,all reactants and products have concentrations equal to 12 M.At equilibrium,the approximate concentration of ammonia is
A) 6 M.
B) 3 M.
C) 12 M.
D) 18 M.
E) 0 M.
Correct Answer:
Verified
Q67: The equilibrium constant K for the
Q75: For the reaction 2NOCl(g) Q76: Consider the reaction CO(g)+ 2H2(g) Q82: Which of the following reactions is not Q153: For the decomposition of ammonia to nitrogen Q167: From a plot of Gibbs free Q170: If a reaction mixture that is Q171: For the decomposition of ammonia to nitrogen Q175: Consider the reaction Q178: For a pure solid or liquid,the molar![]()

2SO2(g)+O2(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents