Consider the reaction CO(g) + 2H2(g) 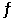 CH3OH(g)
CH3OH(g)
At room temperature,K is approximately 2*104,but at a higher temperature K is substantially smaller.Which of the following is true?
A) The reaction is endothermic.
B) The value of Kc for this reaction is smaller at all temperatures.
C) At the higher temperature, more CH3OH(g) is produced.
D) The reaction is exothermic.
E) The reaction becomes spontaneous at higher temperatures.
Correct Answer:
Verified
Q67: The equilibrium constant K for the
Q71: Consider the reaction 2HI(g) Q75: For the reaction 2NOCl(g) Q77: Consider the reaction 4NH3(g)+ 3O2(g) Q153: For the decomposition of ammonia to nitrogen Q167: From a plot of Gibbs free Q170: If a reaction mixture that is Q171: For the decomposition of ammonia to nitrogen Q175: Consider the reaction Q178: For a pure solid or liquid,the molar![]()
![]()

2SO2(g)+O2(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents