Multiple Choice
Consider the reaction 2HI(g) 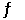 H2(g) + I2(g)
H2(g) + I2(g)
At 298 K,Kc = 1.3 *10-3,whereas at 783 K,Kc = 2.2 *10-2.Which of the following is true?
A) The reaction is exothermic.
B) The reaction is endothermic.
C) At 298 K, K = 3.2 *10-2.
D) At 298 K, the reaction is likely to be spontaneous.
E) At 783 K, more HI(g) is produced.
Correct Answer:
Verified
Related Questions
Q66: Consider the reaction below: Q67: If the equilibrium constant for the reaction Q68: Consider the reaction Q69: Consider the following Q70: For the reaction N2O4(g) Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents
F2(g) ![]()
4NH3(g)+ 7O2(g) ![]()
N2O4(g) ![]()


