Consider the reaction below:
F2(g) 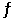 2F(g)
2F(g)
(a)Compressing the reaction mixture results in a change in Q.True or false?
(b)Heating the reaction mixture causes the reaction to shift to the left.True or false?
(c)At 1000 K,the equilibrium constant for the reaction is about 10 - 4.If the reaction is perturbed such that Q = 1,the reaction must shift to the left.True or false?
Correct Answer:
Verified
(...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q61: Consider the reaction 4NH3(g)+ 3O2(g) 
Q62: For the reaction 2NOCl(g) Q63: The vapor pressure of acetic acid Q65: Consider the reaction Ni(s)+ 4CO(g) Q67: If the equilibrium constant for the reaction Q68: Consider the reaction Q69: Consider the following Q70: For the reaction N2O4(g) Q71: Consider the reaction 2HI(g) Q163: For any reaction at equilibrium, Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
4NH3(g)+ 7O2(g) ![]()
N2O4(g) ![]()

![]()

