Consider the reaction 4NH3(g) + 3O2(g) 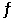 2N2(g) + 6H2O(g) ,K = 1080 at a certain temperature.
2N2(g) + 6H2O(g) ,K = 1080 at a certain temperature.
Initially,all reactants and products have concentrations equal to 12 M.At equilibrium,the approximate concentration of oxygen is
A) 6 M.
B) 0 M.
C) 3 M.
D) 12 M.
E) 18 M.
Correct Answer:
Verified
Q56: Consider the reaction PCl5(g) Q57: The equilibrium constant,Kc,for the reaction 2SO2(g)+ O2(g) Q58: The effect of a volume decrease on Q59: Write the equilibrium constant for 2NaBr(aq)+ Pb(ClO4)2(aq) Q60: Consider the reaction 3Fe(s)+ 4H2O(g) Q62: For the reaction 2NOCl(g) Q63: The vapor pressure of acetic acid Q65: Consider the reaction Ni(s)+ 4CO(g) Q66: Consider the reaction below: Q163: For any reaction at equilibrium, Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()

![]()
F2(g) ![]()

