Consider the Reaction Ni(s)+ 4CO(g) Ni(CO)4(g)
at 30 C and PCO = 1 Atm,Ni Reacts with CO(g)to Form
Consider the reaction Ni(s) + 4CO(g) 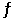 Ni(CO) 4(g)
Ni(CO) 4(g)
At 30 C and PCO = 1 atm,Ni reacts with CO(g) to form Ni(CO) 4(g) .At 200 C,Ni(CO) 4(g) decomposes to Ni(s) and CO(g) .This means
A) adding an inert gas like argon favors the forward reaction.
B) the activation energy for the forward reaction is greater than for the reverse reaction.
C) the forward reaction is endothermic.
D) K at 30 C is greater than K at 200 C.
E) a decrease in pressure favors the forward reaction.
Correct Answer:
Verified
Q60: Consider the reaction 3Fe(s)+ 4H2O(g) 
Q61: Consider the reaction 4NH3(g)+ 3O2(g) 
Q62: For the reaction 2NOCl(g) Q63: The vapor pressure of acetic acid Q66: Consider the reaction below: Q67: If the equilibrium constant for the reaction Q68: Consider the reaction Q69: Consider the following Q70: For the reaction N2O4(g) Q163: For any reaction at equilibrium, Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
F2(g) ![]()
4NH3(g)+ 7O2(g) ![]()
N2O4(g) ![]()


