The phase diagram for a pure substance is shown below. 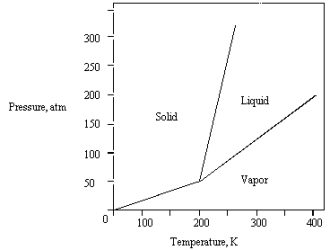 What is the lowest temperature at which liquid can exist?
What is the lowest temperature at which liquid can exist?
A) 400 K
B) 0 K
C) 200 K
D) 150 K
E) 250 K
Correct Answer:
Verified
Q1: In a pressure cooker,the boiling point
Q2: Estimate the enthalpy of vaporization of
Q4: A plot of ln(vapor pressure) versus 1/T
Q5: The phase diagram for a pure compound
Q6: The vapor pressure of water above
Q7: Estimate the enthalpy of vaporization of
Q8: In a closed vessel containing water the
Q9: The vapor pressure of benzene at
Q10: The vapor pressure of methanol at
Q11: A plot of ln(vapor pressure) versus 1/T
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents