Use the following diagram of a cell to answer questions 59-64: 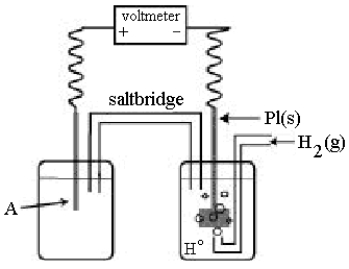
-In the cell shown above, A is a standard Zn2+/Zn electrode connected to a standard hydrogen electrode (SHE) .If the voltmeter reading is -0.76 V,
Which half-reaction occurs in the left-hand cell compartment?
A) Zn2+(aq) + 2e- Zn(s)
B) Zn(s) Zn2+(aq) + 2e-
Correct Answer:
Verified
Q250: The products of the electrolysis of CuSO4(aq)
Q251: Use the following to answer questions 55-58:
Q252: How long will it take to deposit
Q253: Of the following metals, which metal would
Q254: How many moles of Cl2(g) are
Q256: Sodium is produced by electrolysis of molten
Q257: Use the following diagram of a
Q258: When a lead-acid battery discharges,sulfuric acid is
Q259: Use the following diagram of a
Q260: Use the following to answer questions 55-58:
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents