Use the following diagram of a cell to answer questions 59-64: 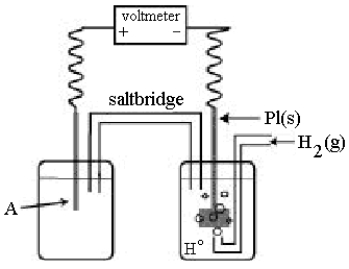
-Using the cell shown above, A is a standard Ag+/Ag electrode connected to a standard hydrogen electrode (SHE) .When the voltmeter reading is -0.80 V,
Which half-reaction occurs in the left-hand cell compartment?
A) Ag(s) Ag+(aq) + e-
B) Ag+(aq) + e- Ag(s)
Correct Answer:
Verified
Q254: How many moles of Cl2(g) are
Q255: Use the following diagram of a
Q256: Sodium is produced by electrolysis of molten
Q257: Use the following diagram of a
Q258: When a lead-acid battery discharges,sulfuric acid is
Q260: Use the following to answer questions 55-58:
Q261: What is E for the half-reaction
Q262: For the cell diagram
Pt|H2(g)|H+(aq)mCo3+(aq),Co2+(aq)|Pt
write the reaction that
Q263: Consider the following cell:
Zn(s)|Zn2+(aq,0.10 M)m Cu2+(aq,0.10 M)|Cu(s)
At
Q264: What are the products of the electrolytic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents