How many moles of magnesium oxide are produced by the reaction of 1.82 g of magnesium nitride with 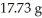 of water?
of water?
Mg3N2 + 3H2O → 2NH3 + 3MgO
A) 0.0180
B) 0.0541
C) 0.00601
D) 0.984
E) 3.00
Correct Answer:
Verified
Q104: GeF3H is formed from GeH4 and GeF4
Q105: Under appropriate conditions,nitrogen and hydrogen undergo a
Q106: Solid aluminum and gaseous oxygen react in
Q107: Under appropriate conditions,nitrogen and hydrogen undergo a
Q108: Sulfur and fluorine react in a combination
Q110: Solid aluminum and gaseous oxygen react in
Q111: What is the maximum number of moles
Q112: What is the maximum mass in grams
Q113: Calcium carbide (CaC2)reacts with water to produce
Q114: Lead (II)carbonate decomposes to give lead (II)oxide
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents