Magnesium and nitrogen react in a combination reaction to produce magnesium nitride: 3 Mg + N2 → Mg3N2
In a particular experiment,a 8.33-g sample of 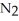 reacts completely.The mass of Mg consumed is ________ g.
reacts completely.The mass of Mg consumed is ________ g.
A) 7.23
B) 21.7
C) 28.9
D) 0.92
E) 13.9
Correct Answer:
Verified
Q141: The combustion of ammonia in the presence
Q142: How many moles of lithium phosphate (Li3PO4)are
Q143: A 1.36-g sample of magnesium nitrate,Mg(NO3)2,contains _
Q144: Magnesium burns in air with a dazzling
Q145: How many grams of calcium cyanide (Ca(CN)2)are
Q147: A certain alcohol contains only three elements,carbon,hydrogen,and
Q148: How many grams of phenol (C6H5OH)are contained
Q149: Lithium and nitrogen react to produce lithium
Q150: Lithium and nitrogen react to produce lithium
Q151: Automotive air bags inflate when sodium azide
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents