For the following reactions,the ΔH°rxn is NOT equal to ΔH°f for the product except for ________.
A) 2Ca (s) + O2 (g) → 2CaO (s)
B) 3Mg (s) + N2 (g) → Mg3N2 (s)
C) C2H2 (g) + H2 (g) → 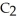 H4 (g)
H4 (g)
D) 2C (graphite) + O2 (g) → 2CO (g)
E) C (diamond) + O2 (g) → CO2 (g)
Correct Answer:
Verified
Q19: When a system _,ΔE is always negative.
A)absorbs
Q20: For a given process at constant pressure,w
Q21: Consider the following two reactions: A →
Q22: Which of the following is a statement
Q23: For the following reactions,the ΔH°rxn is NOT
Q25: Of the following,ΔH°f is not zero for
Q26: All of the following are considered fossil
Q27: The units of specific heat are _.
A)K/J
Q28: For the combustion reaction of methane,ΔH°f is
Q29: For which one of the following reactions
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents