A fixed amount of gas at 25.0 °C occupies a volume of 10.0 L when the pressure is 751 torr.Use Boyle's law to calculate the pressure (torr) when the volume is reduced to 7.25 L at a constant temperature of 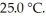
A) 1.04 × 103
B) 0.097
C) 5.44 × 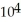
D) 544
E) 1.36
Correct Answer:
Verified
Q107: CO (5.00 g)and CO2 (5.00 g)were placed
Q108: A closed-end manometer was attached to a
Q109: A sample of CO2 gas (3.0 mol)effused
Q110: The root-mean-square speed of CO at 113
Q111: CO (5.00 g)and CO2 (5.00 g)were placed
Q113: Molecular compounds of low molecular weight tend
Q114: A mixture of He and Ne at
Q115: Sodium hydride reacts with excess water to
Q116: A gas mixture of N2 and H2
Q117: SO2 (5.00 g)and CO2 (5.00 g)were placed
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents