The concentration of lead nitrate (Pb(N 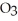
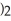 ) in a 0.926 M solution is ________ molal.The density of the solution is 1.202 g/mL.
) in a 0.926 M solution is ________ molal.The density of the solution is 1.202 g/mL.
A) 0.770
B) 2.13
C) 1.03
D) 0.819
E) 0.650
Correct Answer:
Verified
Q99: A solution is prepared by dissolving 2.60
Q100: On a clear day at sea level,with
Q101: What is the molarity of a 7.00%
Q102: 13.3 g of benzene (C6H6)is dissolved in
Q103: The mole fraction of He in a
Q105: A solution contains 30 ppm of some
Q106: What is the molal concentration of a
Q107: What is the mole fraction of hydrochloric
Q108: What is the molarity of phosphoric acid
Q109: What is the mole fraction of N
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents