What is the mole fraction of N 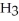 in a solution prepared by dissolving 16.0 g of N
in a solution prepared by dissolving 16.0 g of N 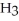 in 250.0 g of water? The density of the resulting solution is 0.974 g/mL.
in 250.0 g of water? The density of the resulting solution is 0.974 g/mL.
A) 0.0640
B) 0.940
C) 0.0635
D) 0.922
E) 16.8
Correct Answer:
Verified
Q104: The concentration of lead nitrate (Pb(N
Q105: A solution contains 30 ppm of some
Q106: What is the molal concentration of a
Q107: What is the mole fraction of hydrochloric
Q108: What is the molarity of phosphoric acid
Q110: Calculate the mole fraction of phosphoric acid
Q111: What is the molality of a 24.4%
Q112: What is the chloride ion concentration (M)in
Q113: What is the molality of a 10.0%
Q114: What is the mole fraction of nitric
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents