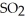
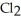 decomposes in the gas phase by the reaction SO2Cl2 (g) → SO2 (g) + Cl2 (g)
decomposes in the gas phase by the reaction SO2Cl2 (g) → SO2 (g) + Cl2 (g)
The reaction is first order in SO2Cl2 and the rate constant is 3.0 × 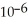
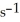 at 600 K.A vessel is charged with 3.6 atm of SO2Cl2 at 600 K.The partial pressure of SO2Cl2 at
at 600 K.A vessel is charged with 3.6 atm of SO2Cl2 at 600 K.The partial pressure of SO2Cl2 at 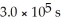 is ________ atm.
is ________ atm.
A) 0.85
B) 3.2
C) 1.5
D) 0.19
E) 9.3 × 104
Correct Answer:
Verified
Q114: A particular first-order reaction has a rate
Q115: The rate of a chemical reaction should
Q116: The Earth's ozone layer is located in
Q117: If a rate law is first order
Q118: Write the rate law for the reaction:
aA
Q120: What is the magnitude of k for
Q121: Units of the rate constant of a
Q122: Rates of reaction can be positive or
Q123: The instantaneous rate of a reaction can
Q124: Define a homogeneous catalyst.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents