A particular first-order reaction has a rate constant of 1.35 × 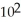
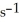 at 25.0 °C.What is the magnitude of k at
at 25.0 °C.What is the magnitude of k at 
A) 1.92 × 103
B) 1.95 × 104
C) 358
D) 3.48 × 1073
E) 1.35 × 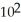
Correct Answer:
Verified
Q109: The decomposition of Q110: According to the collision model,reaction rates are Q111: The rate constant for a particular second-order Q112: The initial concentration of reactant in a Q113: Define activation energy. Q115: The rate of a chemical reaction should Q116: The Earth's ozone layer is located in Q117: If a rate law is first order Q118: Write the rate law for the reaction: Q119: ![]()
aA![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents