Consider the following chemical reaction: CO (g) + 2H2(g)  CH3OH(g)
CH3OH(g)
At equilibrium in a particular experiment,the concentrations of CO and H2 were 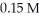 and
and 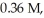 respectively.What is the equilibrium concentration of CH3OH?
respectively.What is the equilibrium concentration of CH3OH?
The value of Keq for this reaction is 14.5 at the temperature of the experiment.
A) 14.5
B) 7.61 × 10-3
C) 2.82 × 10-1
D) 3.72 × 10-3
E) 1.34 × 10-3
Correct Answer:
Verified
Q50: Given the following reaction at equilibrium,if Kc
Q51: Which of the following expressions is the
Q52: The value of Keq for the equilibrium
Q53: The Keq for the equilibrium below is
Q54: The Keq for the equilibrium below is
Q56: The value of Keq for the equilibrium
Q57: A sealed 1.0 L flask is charged
Q58: Acetic acid is a weak acid that
Q59: Given the following reaction at equilibrium at
Q60: The value of Keq for the equilibrium
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents