A sealed 1.0 L flask is charged with 0.500 mol of I2 and 0.500 mol of Br2.An equilibrium reaction ensues: I2 (g) + Br2 (g)  2IBr (g)
2IBr (g)
When the container contents achieve equilibrium,the flask contains 0.84 mol of IBr.The value of 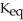 is ________.
is ________.
A) 11
B) 4.0
C) 110
D) 6.1
E) 2.8
Correct Answer:
Verified
Q52: The value of Keq for the equilibrium
Q53: The Keq for the equilibrium below is
Q54: The Keq for the equilibrium below is
Q55: Consider the following chemical reaction: CO (g)+
Q56: The value of Keq for the equilibrium
Q58: Acetic acid is a weak acid that
Q59: Given the following reaction at equilibrium at
Q60: The value of Keq for the equilibrium
Q61: Consider the following reaction at equilibrium: 2C
Q62: The reaction below is exothermic: 2SO2 (g)+
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents