For the endothermic reaction CaCO3 (s)  CaO (s) + CO2 (g)
CaO (s) + CO2 (g)
Le Châtelier's principle predicts that ________ will result in an increase in the number of moles of 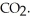
A) increasing the temperature
B) decreasing the temperature
C) increasing the pressure
D) removing some of the CaCO3(s)
E) none of the above
Correct Answer:
Verified
Q62: The reaction below is exothermic: 2SO2 (g)+
Q63: At elevated temperatures,molecular hydrogen and molecular bromine
Q64: The Keq for the equilibrium below is
Q65: Q66: Phosphorous trichloride and phosphorous pentachloride equilibrate in Q68: The value of Keq for the following Q69: In the coal-gasification process,carbon monoxide reacts with Q70: Hydrogen and iodine gas react to produce Q71: At 24°C,Kp = 0.080 for the equilibrium: Q72: Dinitrogen tetroxide partially decomposes according to the![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents