The hydride ion, 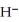 ,is a stronger base than the hydroxide ion,O
,is a stronger base than the hydroxide ion,O 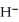 .The product(s) of the reaction of hydride ion with water is/are ________.
.The product(s) of the reaction of hydride ion with water is/are ________.
A) H3O+ (aq)
B) OH- (aq) + H2 (g)
C) OH- (aq) + 2H+ (aq)
D) no reaction occurs
E) H2O2 (aq)
Correct Answer:
Verified
Q1: According to the Arrhenius concept,an acid is
Q2: The molar concentration of hydronium ion in
Q3: Which one of the following statements regarding
Q4: Of the acids in the table below,_
Q6: Of the following acids,_ is a strong
Q7: The Ka of hypochlorous acid (HClO)is 3.0
Q8: The molar concentration of hydroxide ion in
Q9: The magnitude of Kw indicates that _.
A)water
Q10: Classify the following compounds as weak acids
Q11: Which one of the following is a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents