Multiple Choice
The Ka of hypochlorous acid (HClO) is 3.0 × 10-8 at 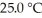 .What is the percent ionization of hypochlorous acid in a
.What is the percent ionization of hypochlorous acid in a 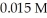 aqueous solution of HClO at
aqueous solution of HClO at 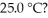
A) 4.5 × 10-8
B) 14
C) 2.1 × 10-5
D) 0.14
E) 1.4 × 10-3
Correct Answer:
Verified
Related Questions
Q2: The molar concentration of hydronium ion in
Q3: Which one of the following statements regarding
Q4: Of the acids in the table below,_
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents