Multiple Choice
What is the pOH of 0.606 M anilinium hydrochloride ( 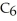
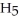 N
N 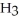 Cl) solution in water,given that
Cl) solution in water,given that 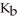 for aniline is 3.83 ×
for aniline is 3.83 × 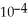 ?
?
A) 12.42
B) 1.82
C) 8.60
D) 5.40
E) 12.18
Correct Answer:
Verified
Related Questions
Q122: What is the pOH of a sodium
Q123: The Q124: The Q125: A 0.045 M solution of ammonia is Q126: What is the pOH of a 0.20 Q128: What is the pH of a sodium Q129: The pH of a 0.25 M aqueous Q130: Of the following,which is the strongest acid? Q131: A Lewis acid is an electron-pair acceptor,and Q132: The Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
A)HIO4
B)HIO3
C)HIO2
D)HIO
E)The![]()

