The concentration of iodide ions in a saturated solution of lead (II) iodide is ________ M.The solubility product constant of PbI2 is 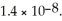
A) 3.8 × 10-4
B) 3.0 × 10-3
C) 1.5 × 10-3
D) 3.5 × 10-9
E) 1.4 × 10-8
Correct Answer:
Verified
Q42: The Ksp for Cu(OH)2 is 4.8 ×
Q43: The solubility of lead (II)chloride (PbCl2)is
Q44: What is the solubility (in M)of PbCl2
Q45: The concentration of iodide ions in a
Q46: Calculate the pH of a solution that
Q48: Calculate the percent ionization of formic acid
Q49: Calculate the maximum concentration (in M)of silver
Q50: What is the percent ionization of nitrous
Q51: A 50.0 mL sample of an aqueous
Q52: The pH of a solution prepared by
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents