The solubility of lead (II) chloride (PbCl2) is 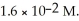 What is the Ksp of PbCl2?
What is the Ksp of PbCl2?
A) 5.0 × 10-4
B) 4.1 × 10-6
C) 3.1 × 10-7
D) 1.6 × 10-5
E) 1.6 × 10-2
Correct Answer:
Verified
Q38: The pH of a solution prepared by
Q39: In which one of the following solutions
Q40: Decreasing the pH of blood will cause
Q41: The pH of a solution prepared by
Q42: The Ksp for Cu(OH)2 is 4.8 ×
Q44: What is the solubility (in M)of PbCl2
Q45: The concentration of iodide ions in a
Q46: Calculate the pH of a solution that
Q47: The concentration of iodide ions in a
Q48: Calculate the percent ionization of formic acid
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents