Multiple Choice
The 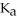 of some weak acid HA is 1.76 ×
of some weak acid HA is 1.76 × 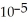 .The pH of a buffer prepared by combining 15.0 mL of
.The pH of a buffer prepared by combining 15.0 mL of 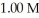 A- and 50.0 mL of 1.00 M HA is ________.
A- and 50.0 mL of 1.00 M HA is ________.
A) 1.705
B) 4.232
C) 0.851
D) 2.383
E) 3.406
Correct Answer:
Verified
Related Questions
Q58: Determine the Ksp for magnesium hydroxide (Mg(OH)2)where
Q59: Calculate the pH of a solution that
Q60: Calculate the maximum concentration (in M)of calcium
Q61: What is the pH of a buffer
Q62: Which solution would have the greatest buffering
Q64: A solution is prepared by dissolving 0.23
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents