Determine the Ksp for magnesium hydroxide (Mg(OH) 2) where the solubility of Mg(OH) 2 is 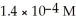 .
.
A) 2.7 × 10-12
B) 1.1 × 10-11
C) 2.0 × 10-8
D) 3.9 × 10-8
E) 1.4 × 10-4
Correct Answer:
Verified
Q53: The concentration of fluoride ions in a
Q54: What is the percent ionization of nitrous
Q55: Calculate the percent ionization of formic acid
Q56: The solubility of manganese (II)hydroxide (Mn(OH)2)is
Q57: The pH of a solution prepared by
Q59: Calculate the pH of a solution that
Q60: Calculate the maximum concentration (in M)of calcium
Q61: What is the pH of a buffer
Q62: Which solution would have the greatest buffering
Q63: The ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents