A 25.0-mL sample of 0.150 M hydrocyanic acid is titrated with a 0.150 M NaOH solution.What is the pH before any base is added? The 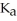 of hydrocyanic acid is 4.9 × 10-10.
of hydrocyanic acid is 4.9 × 10-10.
A) 5.07
B) 8.6 × 10-6
C) 9.31
D) 8.49
E) 3.1 × 108
Correct Answer:
Verified
Q77: A solution is prepared by dissolving 0.23
Q78: What is the pH of a buffer
Q79: Calculate the pH of a solution prepared
Q80: A buffer solution with a pH of
Q81: What is the pH of a solution
Q83: A 25.0 mL sample of 0.150 M
Q84: A 25.0 mL sample of 0.723 M
Q85: In which of the following aqueous solutions
Q86: In which of the following aqueous solutions
Q87: AgBr would have the lowest solubility in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents