A 25.0 mL sample of 0.723 M HCl 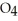 is titrated with a
is titrated with a 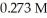 KOH solution.The
KOH solution.The 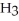
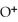 concentration after the addition of
concentration after the addition of 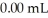 of KOH is ________ M.
of KOH is ________ M.
A) 0.0181
B) 0.430
C) 0.723
D) 0.273
E) none of the above
Correct Answer:
Verified
Q79: Calculate the pH of a solution prepared
Q80: A buffer solution with a pH of
Q81: What is the pH of a solution
Q82: A 25.0-mL sample of 0.150 M hydrocyanic
Q83: A 25.0 mL sample of 0.150 M
Q85: In which of the following aqueous solutions
Q86: In which of the following aqueous solutions
Q87: AgBr would have the lowest solubility in
Q88: What is the molar solubility of silver
Q89: Which is the correct ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents