A 25.0-mL sample of 0.150 M hypochlorous acid is titrated with a 0.150 M NaOH solution.What is the pH after 13.3 mL of base is added? The 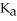 of hypochlorous acid is 3.0 × 10-8.
of hypochlorous acid is 3.0 × 10-8.
A) 7.25
B) 1.34
C) 4.43
D) 7.58
E) 7.46
Correct Answer:
Verified
Q89: Which is the correct Q90: How many milliliters of 0.120 M NaOH Q91: In which aqueous system is Q92: What is the molar solubility of calcium Q93: The pH of a solution prepared by Q95: Of the substances below,_ will decrease the Q96: A 25.0 mL sample of an acetic Q97: A 25.0 mL sample of an HCl Q98: In which of the following aqueous solutions Q99: In which of the following aqueous solutions![]()

Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents