The pH of a solution prepared by mixing 45.0 mL of 0.183 M KOH and 35.0 mL of 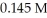 HCl is ________.
HCl is ________.
A) 1.314
B) 1.403
C) 0.00824
D) 12.597
E) 12.923
Correct Answer:
Verified
Q88: What is the molar solubility of silver
Q89: Which is the correct Q90: How many milliliters of 0.120 M NaOH Q91: In which aqueous system is Q92: What is the molar solubility of calcium Q94: A 25.0-mL sample of 0.150 M hypochlorous Q95: Of the substances below,_ will decrease the Q96: A 25.0 mL sample of an acetic Q97: A 25.0 mL sample of an HCl Q98: In which of the following aqueous solutions![]()

Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents