Consider the reaction between ammonia and hydrochloric acid to produce ammonium chloride. Given the following table of thermodynamic data at 298 K: 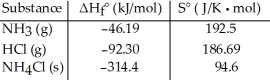 The value of K for the reaction at 25 °C is ________.
The value of K for the reaction at 25 °C is ________.
A) 8.4 × 104
B) 150
C) 1.1 × 10-16
D) 9.3 × 1015
E) 1.4 × 108
Correct Answer:
Verified
Q91: The standard Gibbs free energy of formation
Q92: Consider the reaction: FeO (s)+ Fe (s)+
Q93: The signs of ΔH° and ΔS° must
Q94: ΔS is negative for the reaction _.
A)Sr(NO3)2
Q95: The standard Gibbs free energy of formation
Q97: Consider the formation of solid silver chloride
Q98: Which of the following has the largest
Q99: The value of ΔG° at 181.0 °C
Q100: What is the equilibrium constant for a
Q101: At what temperature (in K)will a reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents