The value of ΔG° at 181.0 °C for the formation of calcium chloride from calcium metal and chlorine gas is ________ kJ/mol.At 25.0 °C for this reaction,ΔH° is -795.8 kJ/mol,ΔG° is -748.1 kJ/mol,and 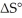 is
is 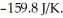
A) 7.18 × 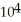
B) -868.4
C) -766.9
D) -723.2
E) 2.81 × 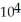
Correct Answer:
Verified
Q94: ΔS is negative for the reaction _.
A)Sr(NO3)2
Q95: The standard Gibbs free energy of formation
Q96: Consider the reaction between ammonia and hydrochloric
Q97: Consider the formation of solid silver chloride
Q98: Which of the following has the largest
Q100: What is the equilibrium constant for a
Q101: At what temperature (in K)will a reaction
Q102: What is the equilibrium constant for a
Q103: A reversible change produces the maximum amount
Q104: In the Haber process,ammonia gas is synthesized
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents