Multiple Choice
For a given reaction,ΔS = +69.0 J/mol∙K,and the reaction is spontaneous at temperatures above the crossover temperature, 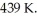 The value of
The value of  assuming that ΔH and ΔS do not vary with temperature.
assuming that ΔH and ΔS do not vary with temperature.
A) 30.3
B) -30.3
C) 1.57 × 10-4
D) -1.57 × 10-4
E) 6.36 × 10-3
Correct Answer:
Verified
Related Questions
Q103: A reversible change produces the maximum amount
Q104: In the Haber process,ammonia gas is synthesized
Q105: For a given reaction with ΔS =
Q106: For a given reaction,ΔH = +74.6 kJ/mol,and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents