At what temperature in Kelvin will a reaction have 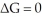 ? ΔH = -24.2 kJ/mol and ΔS = -55.5 J/K-mol and assume both do not vary with temperature.
? ΔH = -24.2 kJ/mol and ΔS = -55.5 J/K-mol and assume both do not vary with temperature.
A) 2.29
B) 2293
C) 298
D) 436
E) 0.436
Correct Answer:
Verified
Q108: For a given reaction,ΔS = +69.0 J/mol∙K,and
Q109: At what temperature (in K)will a reaction
Q110: The equilibrium constant for a reaction is
Q111: If ΔG° for a reaction is less
Q112: Calculate ΔG∘ (in kJ/mol)for the following reaction
Q114: What is the ΔG° (kJ/mol)for the formation
Q115: Phosphorous and chlorine gases combine to produce
Q116: At what temperature will a reaction be
Q117: The equilibrium constant for the following reaction
Q118: Given the following table of thermodynamic data,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents