The equilibrium constant for the following reaction is 3.0 × 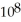 at 25 °C.
at 25 °C. 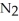 (g) + 3
(g) + 3 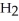 (g)
(g)  2N
2N 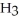 (g) The value of ΔG° for this reaction is ________ kJ/mol.
(g) The value of ΔG° for this reaction is ________ kJ/mol.
A) 22
B) -4.1
C) 4.1
D) -48
E) -22
Correct Answer:
Verified
Q112: Calculate ΔG∘ (in kJ/mol)for the following reaction
Q113: At what temperature in Kelvin will a
Q114: What is the ΔG° (kJ/mol)for the formation
Q115: Phosphorous and chlorine gases combine to produce
Q116: At what temperature will a reaction be
Q118: Given the following table of thermodynamic data,
Q119: Calculate ΔG° (in kJ/mol)for the following reaction
Q120: For a given reaction with ΔH =
Q121: The entropy of a pure crystalline substance
Q122: The melting of a substance at its
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents