In the reaction 16 H+(aq) + 10 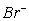 (aq) + 2
(aq) + 2 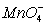 (aq) → 5 Br2(l) + 2 Mn2+(aq) + 8 H2O(l)
(aq) → 5 Br2(l) + 2 Mn2+(aq) + 8 H2O(l)
The reactants are:
A) H+(aq) , 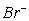 (aq) ,
(aq) ,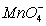 (aq)
(aq)
B) Br2(l) ,Mn2+(aq) ,H2O(l)
C) H+(aq) ,H2O(l)
D) 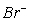 (aq) ,
(aq) ,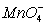 (aq)
(aq)
Correct Answer:
Verified
Q66: When 20.0g of mercury is heated from
Q67: The following list represents properties of iron
Q68: A shiny silver colored strip of metal
Q69: Which phase change releases energy?
A)Gas to liquid
B)Solid
Q70: The specific heat of copper is 0.386
Q72: How much heat energy is required to
Q73: How many kiloJoules of heat energy are
Q74: Which of the following represents a physical
Q75: A sample of an unknown substance was
Q76: As the average kinetic energy of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents