How many grams of O2 are required to produce 1.23 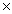 1024 molecules of water? 2H2 + O2
1024 molecules of water? 2H2 + O2  2H2O
2H2O
A) 32.7 g
B) 131 g
C) 65.4 g
D) 16.3 g
Correct Answer:
Verified
Q2: How many moles of benzene (C6H6)should be
Q3: How many grams of sodium iodide are
Q4: What mass of sodium chloride will be
Q5: How many grams of water will be
Q6: What mass of sodium hydroxide will neutralize
Q8: How many molecules of O2 will be
Q9: Calculate the number of moles of excess
Q10: Calculate the number of moles of excess
Q11: How many units of sodium iodide will
Q12: How many moles of NH3 will
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents