Calculate the number of moles of excess reactant that will be left-over when 0.350 g of HCN react with 0.500 g of O2: 4HCN + 5O2  2N2 + 4CO2 + 2H2O
2N2 + 4CO2 + 2H2O
A) 0.150 moles
B) 3.24  10
10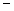
3 moles
C) 4.50 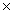 10
10
4 moles
D) 5.62 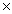 10
10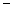
4 moles
Correct Answer:
Verified
Q12: How many moles of NH3 will
Q13: How many moles of HF will
Q14: How many moles of iron(III)oxide are required
Q15: How many molecules of O2 will be
Q16: How many moles of molecular oxygen will
Q18: Based on the following chemical equation 4HCN
Q19: Based on the following chemical equation 2C6H6
Q20: What mass of sodium hydroxide will be
Q21: How many moles of H2O are
Q22: How many units of K2SO4 are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents