Consider the single-displacement reaction in which zinc metal displaces hydrogen from hydrochloric acid.
A.Write a complete and balanced chemical equation for this reaction.
B.If 3.4
 1023 zinc atoms react with 4.5
1023 zinc atoms react with 4.5
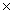 1023 molecules of hydrochloric acid,calculate the mass of zinc chloride produced in this reaction.
1023 molecules of hydrochloric acid,calculate the mass of zinc chloride produced in this reaction.
C.Calculate the number of moles of the excess reactant that remain once the reaction stops.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q91: The limiting reactant in a chemical reaction
Q92: The limiting reactant in a chemical reaction
Q93: Nitrogen gas combines with hydrogen gas to
Q94: A mole ratio is used to convert
Q95: Nitrogen gas to water
Q96: In every chemical reaction the number of
Q97: Sulfur dioxide reacts with oxygen to produce
Q98: The equation for a reaction should be
Q99: Two solutions are combined in a beaker.One
Q100: Answer the following questions based on the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents