In terms of a0,where a0 = 0.0529 nm,the radii of the allowed orbits in the Bohr model of the hydrogen atom are given by rn =
A) 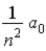 .
.
B) 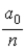 .
.
C) 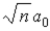 .
.
D) na0.
E) n2a0.
Correct Answer:
Verified
Q36: For the following allowed transitions, which photon
Q36: The magnitude of the spin angular momentum
Q37: The probability density for the 1s state
Q38: The ground state configuration of chlorine (Z
Q39: If P(r)is the radial probability density function
Q42: The number of electrons in the n
Q43: Adam and Eve are contemplating the beauty
Q44: In the Bohr model of the hydrogen
Q45: Which of the following,in which n and
Q46: In an allowed electron transition in a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents