The number of electrons in the n = 4, 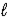
= 2 subshell in strontium (Z = 38) is ____ the number of electrons in the n =4, 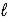
=2 subshell in barium (Z = 56) .
A) 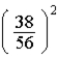 times
times
B) 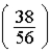 times
times
C) equal to
D) 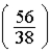 times
times
E) 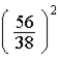 times
times
Correct Answer:
Verified
Q36: For the following allowed transitions, which photon
Q37: The probability density for the 1s state
Q38: The ground state configuration of chlorine (Z
Q39: If P(r)is the radial probability density function
Q41: In terms of a0,where a0 = 0.0529
Q43: Adam and Eve are contemplating the beauty
Q44: In the Bohr model of the hydrogen
Q45: Which of the following,in which n and
Q46: In an allowed electron transition in a
Q47: The energy difference between the upper and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents