When a molecule jumps from a rotational energy level characterized by the rotational quantum number J to one characterized by J − 1,the difference in energy of levels J and J − 1,EJ − EJ − 1,is
A) 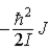 .
.
B) 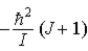 .
.
C) 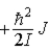 .
.
D) 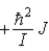 .
.
E) 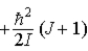 .
.
Correct Answer:
Verified
Q19: An oxygen molecule has a moment of
Q20: Assume the angular momentum of a diatomic
Q23: In the Lennard-Jones model of the hydrogen
Q27: If an electric field is applied to
Q27: The wave functions of some molecules are
Q28: In comparing vibrational and rotational levels in
Q29: When a voltage ΔV is applied to
Q30: Because HF, hydrogen fluoride, is a covalent
Q32: The difference between donor and acceptor atoms
Q39: The energy of a molecule can normally
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents